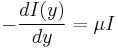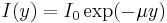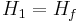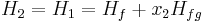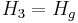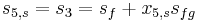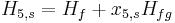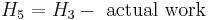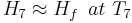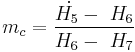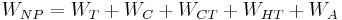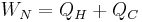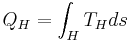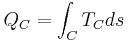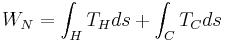Ocean thermal energy conversion
Ocean Thermal Energy Conversion (OTEC) uses the difference between cooler deep and warmer shallow or surface ocean waters to run a heat engine and produce useful work, usually in the form of electricity.
A heat engine gives greater efficiency and power when run with a large temperature difference. In the oceans the temperature difference between surface and deep water is greatest in the tropics, although still a modest 20 to 25 °C. It is therefore in the tropics that OTEC offers the greatest possibilities.[1] OTEC has the potential to offer global amounts of energy that are 10 to 100 times greater than other ocean energy options such as wave power. OTEC plants can operate continuously providing a base load supply for an electrical power generation system.[1]
The main technical challenge of OTEC is to generate significant amounts of power efficiently from small temperature differences. It is still considered an emerging technology. Early OTEC systems were of 1 to 3% thermal efficiency, well below the theoretical maximum for this temperature difference of between 6 and 7%.[2] Current designs are expected to be closer to the maximum. The first operational system was built in Cuba in 1930 and generated 22 kW. Modern designs allow performance approaching the theoretical maximum Carnot efficiency and the largest built in 1999 by the USA generated 250 kW .
The most commonly used heat cycle for OTEC is the Rankine cycle using a low-pressure turbine. Systems may be either closed-cycle or open-cycle. Closed-cycle engines use working fluids that are typically thought of as refrigerants such as ammonia or R-134a. Open-cycle engines use vapour from the seawater itself as the working fluid.
OTEC can also supply quantities of cold water as a by-product . This can be used for air conditioning and refrigeration and the fertile deep ocean water can feed biological technologies. Another by-product is fresh water distilled from the sea.[1]
Contents |
History
Attempts to develop and refine OTEC technology started in the 1880s. In 1881, Jacques Arsene d'Arsonval, a French physicist, proposed tapping the thermal energy of the ocean. D'Arsonval's student, Georges Claude, built the first OTEC plant, in Matanzas, Cuba in 1930.[3][4] The system generated 22 kW of electricity with a low-pressure turbine.[5]
In 1931, Nikola Tesla released "Our Future Motive Power", which described such a system.[6] Tesla ultimately concluded that the scale of engineering required made it impractical for large scale development.
In 1935, Claude constructed a plant aboard a 10,000-ton cargo vessel moored off the coast of Brazil. Weather and waves destroyed it before it could generate net power.[5] (Net power is the amount of power generated after subtracting power needed to run the system.)
In 1956, French scientists designed a 3 MW plant for Abidjan, Ivory Coast. The plant was never completed, because new finds of large amounts of cheap oil made it uneconomical.[5]
In 1962, J. Hilbert Anderson and James H. Anderson, Jr. focused on increasing component efficiency. They patented their new "closed cycle" design in 1967.[7]
Japan is a major contributor to the development of the technology.[8] Beginning in 1970 the Tokyo Electric Power Company successfully built and deployed a 100 kW closed-cycle OTEC plant on the island of Nauru.[8] The plant became operational 1981-10-14, producing about 120 kW of electricity; 90 kW was used to power the plant and the remaining electricity was used to power a school and other places.[5] This set a world record for power output from an OTEC system where the power was sent to a real power grid.[9] Currently, the Institue of Ocean Energy, Saga University, is the leader and focuses on the power cycle and many of the secondary benifits.
The United States became involved in 1974, establishing the Natural Energy Laboratory of Hawaii Authority at Keahole Point on the Kona coast of Hawaiʻi. Hawaii is the best U.S. OTEC location, due to its warm surface water, access to very deep, very cold water, and Hawaii's high electricity costs. The laboratory has become a leading test facility for OTEC technology.[10]
India built a one MW floating OTEC pilot plant near Tamil Nadu, and its government continues to sponsor research.[11]
Cycle types
Cold seawater is an integral part of each of the three types of OTEC systems: closed-cycle, open-cycle, and hybrid. To operate, the cold seawater must be brought to the surface. The primary approaches are active pumping and desalination. Desalinating seawater near the sea floor lowers its density, which causes it to rise to the surface.[12]
The alternative to costly pipes to bring condensing cold water to the surface is to pump vaporized low boiling point fluid into the depths to be condensed, thus reducing pumping volumes and reducing technical and environmental problems and lowering costs.
Closed
Closed-cycle systems use fluid with a low boiling point, such as ammonia, to power a turbine to generate electricity. Warm surface seawater is pumped through a heat exchanger to vaporize the fluid. The expanding vapor turns the turbo-generator. Cold water, pumped through a second heat exchanger, condenses the vapor into a liquid, which is then recycled through the system.
In 1979, the Natural Energy Laboratory and several private-sector partners developed the "mini OTEC" experiment, which achieved the first successful at-sea production of net electrical power from closed-cycle OTEC.[13] The mini OTEC vessel was moored 1.5 miles (2.4 km) off the Hawaiian coast and produced enough net electricity to illuminate the ship's light bulbs and run its computers and television.
Open
Open-cycle OTEC uses warm surface water directly to make electricity. Placing warm seawater in a low-pressure container causes it to boil. The expanding steam drives a low-pressure turbine attached to an electrical generator. The steam, which has left its salt and other contaminants in the low-pressure container, is pure fresh water. It is condensed into a liquid by exposure to cold temperatures from deep-ocean water. This method produces desalinized fresh water, suitable for drinking water or irrigation.[14]
In 1984, the Solar Energy Research Institute (now the National Renewable Energy Laboratory) developed a vertical-spout evaporator to convert warm seawater into low-pressure steam for open-cycle plants. Conversion efficiencies were as high as 97% for seawater-to-steam conversion (overall efficiency using a vertical-spout evaporator would still only be a few per cent). In May 1993, an open-cycle OTEC plant at Keahole Point, Hawaii, produced 50,000 watts of electricity during a net power-producing experiment.[15] This broke the record of 40 kW set by a Japanese system in 1982.[15]
Hybrid
A hybrid cycle combines the features of the closed- and open-cycle systems. In a hybrid, warm seawater enters a vacuum chamber and is flash-evaporated, similar to the open-cycle evaporation process. The steam vaporizes the ammonia working fluid of a closed-cycle loop on the other side of an ammonia vaporizer. The vaporized fluid then drives a turbine to produce electricity. The steam condenses within the heat exchanger and provides desalinated water. (see heat pipe)
Working fluids
A popular choice of working fluid is ammonia, which has superior transport properties, easy availability, and low cost. Ammonia, however, is toxic and flammable. Fluorinated carbons such as CFCs and HCFCs are not toxic or flammable, but they contribute to ozone layer depletion. Hydrocarbons too are good candidates, but they are highly flammable; in addition, this would create competition for use of them directly as fuels. The power plant size is dependent upon the vapor pressure of the working fluid. With increasing vapor pressure, the size of the turbine and heat exchangers decreases while the wall thickness of the pipe and heat exchangers increase to endure high pressure especially on the evaporator side.
Land, shelf and floating sites
OTEC has the potential to produce gigawatts of electrical power, and in conjunction with electrolysis, could produce enough hydrogen to completely replace all projected global fossil fuel consumption. Reducing costs remains an unsolved challenge, however. OTEC plants require a long, large diameter intake pipe, which is submerged a kilometer or more into the ocean's depths, to bring cold water to the surface.
Land-based
Land-based and near-shore facilities offer three main advantages over those located in deep water. Plants constructed on or near land do not require sophisticated mooring, lengthy power cables, or the more extensive maintenance associated with open-ocean environments. They can be installed in sheltered areas so that they are relatively safe from storms and heavy seas. Electricity, desalinated water, and cold, nutrient-rich seawater could be transmitted from near-shore facilities via trestle bridges or causeways. In addition, land-based or near-shore sites allow plants to operate with related industries such as mariculture or those that require desalinated water.
Favored locations include those with narrow shelves (volcanic islands), steep (15-20 degrees) offshore slopes, and relatively smooth sea floors. These sites minimize the length of the intake pipe. A land-based plant could be built well inland from the shore, offering more protection from storms, or on the beach, where the pipes would be shorter. In either case, easy access for construction and operation helps lower costs.
Land-based or near-shore sites can also support mariculture. Tanks or lagoons built on shore allow workers to monitor and control miniature marine environments. Mariculture products can be delivered to market via standard transport.
One disadvantage of land-based facilities arises from the turbulent wave action in the surf zone. Unless the OTEC plant's water supply and discharge pipes are buried in protective trenches, they will be subject to extreme stress during storms and prolonged periods of heavy seas. Also, the mixed discharge of cold and warm seawater may need to be carried several hundred meters offshore to reach the proper depth before it is released. This arrangement requires additional expense in construction and maintenance.
OTEC systems can avoid some of the problems and expenses of operating in a surf zone if they are built just offshore in waters ranging from 10 to 30 meters deep (Ocean Thermal Corporation 1984). This type of plant would use shorter (and therefore less costly) intake and discharge pipes, which would avoid the dangers of turbulent surf. The plant itself, however, would require protection from the marine environment, such as breakwaters and erosion-resistant foundations, and the plant output would need to be transmitted to shore.
Shelf-based
To avoid the turbulent surf zone as well as to move closer to the cold-water resource, OTEC plants can be mounted to the continental shelf at depths up to 100 meters (330 ft). A shelf-mounted plant could be towed to the site and affixed to the sea bottom. This type of construction is already used for offshore oil rigs. The complexities of operating an OTEC plant in deeper water may make them more expensive than land-based approaches. Problems include the stress of open-ocean conditions and more difficult product delivery. Addressing strong ocean currents and large waves adds engineering and construction expense. Platforms require extensive pilings to maintain a stable base. Power delivery can require long underwater cables to reach land. For these reasons, shelf-mounted plants are less attractive.
Floating
Floating OTEC facilities operate off-shore. Although potentially optimal for large systems, floating facilities present several difficulties. The difficulty of mooring plants in very deep water complicates power delivery. Cables attached to floating platforms are more susceptible to damage, especially during storms. Cables at depths greater than 1000 meters are difficult to maintain and repair. Riser cables, which connect the sea bed and the plant, need to be constructed to resist entanglement.
As with shelf-mounted plants, floating plants need a stable base for continuous operation. Major storms and heavy seas can break the vertically suspended cold-water pipe and interrupt warm water intake as well. To help prevent these problems, pipes can be made of flexible polyethylene attached to the bottom of the platform and gimballed with joints or collars. Pipes may need to be uncoupled from the plant to prevent storm damage. As an alternative to a warm-water pipe, surface water can be drawn directly into the platform; however, it is necessary to prevent the intake flow from being damaged or interrupted during violent motions caused by heavy seas.
Connecting a floating plant to power delivery cables requires the plant to remain relatively stationary. Mooring is an acceptable method, but current mooring technology is limited to depths of about 2,000 meters (6,600 ft). Even at shallower depths, the cost of mooring may be prohibitive.
Some proposed projects
OTEC projects under consideration include a small plant for the U.S. Navy base on the British overseas territory island of Diego Garcia in the Indian Ocean. OCEES International, Inc. is working with the U.S. Navy on a design for a proposed 13-MW OTEC plant, to replace the current diesel generators. The OTEC plant would also provide 1.25 million gallons per day (MGD) of potable water. A private U.S. company has proposed building a 10-MW OTEC plant on Guam.
Hawaii
Lockheed Martin's Alternative Energy Development team has partnered with Makai Ocean Engineering [16] to complete the final design phase of a 10-MW closed cycle OTEC pilot system which will become operational in Hawaii in the 2012-2013 time frame. This system is being designed to expand to 100-MW commercial systems in the near future. In November, 2010 the U.S. Naval Facilities Engineering Command (NAVFAC) awarded Lockheed Martin a US$4.4 million contract modification to develop critical system components and designs for the plant, adding to the 2009 $8.1 million contract and two Department of Energy grants totaling $1 million in 2008 and March 2010.[17]
Related activities
OTEC has uses other than power production.
Air conditioning
The 41 °F (5 °C) cold seawater made available by an OTEC system creates an opportunity to provide large amounts of cooling to operations near the plant. The water can be used in chilled-water coils to provide air-conditioning for buildings. It is estimated that a pipe 1 foot (0.30 m) in diameter can deliver 4,700 gallons per minute of water. Water at 43 °F (6 °C) could provide more than enough air-conditioning for a large building. Operating 8,000 hours per year in lieu of electrical conditioning selling for 5-10¢ per kilowatt-hour, it would save $200,000-$400,000 in energy bills annually.[18]
The InterContinental Resort and Thalasso-Spa on the island of Bora Bora uses an OTEC system to air-condition its buildings.[19] The system passes seawater through a heat exchanger where it cools freshwater in a closed loop system. This freshwater is then pumped to buildings and directly cools the air.
Chilled-soil agriculture
OTEC technology supports chilled-soil agriculture. When cold seawater flows through underground pipes, it chills the surrounding soil. The temperature difference between roots in the cool soil and leaves in the warm air allows plants that evolved in temperate climates to be grown in the subtropics. Dr. John P. Craven, Dr. Jack Davidson and Richard Bailey patented this process and demonstrated it at a research facility at the Natural Energy Laboratory of Hawaii Authority (NELHA).[20] The research facility demonstrated that more than 100 different crops can be grown using this system. Many normally could not survive in Hawaii or at Keahole Point.
Aquaculture
Aquaculture is the best-known byproduct, because it reduces the financial and energy costs of pumping large volumes of water from the deep ocean. Deep ocean water contains high concentrations of essential nutrients that are depleted in surface waters due to biological consumption. This "artificial upwelling" mimics the natural upwellings that are responsible for fertilizing and supporting the world's largest marine ecosystems, and the largest densities of life on the planet.
Cold-water delicacies, such as salmon and lobster, thrive in this nutrient-rich, deep, seawater. Microalgae such as Spirulina, a health food supplement, also can be cultivated. Deep-ocean water can be combined with surface water to deliver water at an optimal temperature.
Non-native species such as Salmon, lobster, abalone, trout, oysters, and clams can be raised in pools supplied by OTEC-pumped water. This extends the variety of fresh seafood products available for nearby markets. Such low-cost refrigeration can be used to maintain the quality of harvested fish, which deteriorate quickly in warm tropical regions.
Desalination
Desalinated water can be produced in open- or hybrid-cycle plants using surface condensers to turn evaporated seawater into potable water. System analysis indicates that a 2-megawatt plant could produce about 4,300 cubic metres (150,000 cu ft) of desalinated water each day.[21] Another system patented by Richard Bailey creates condensate water by regulating deep ocean water flow through surface condensers correlating with fluctuating dew-point temperatures.[22] This condensation system uses no incremental energy and has no moving parts.
Hydrogen production
Hydrogen can be produced via electrolysis using OTEC electricity. Generated steam with electrolyte compounds added to improve efficiency is a relatively pure medium for hydrogen production. OTEC can be scaled to generate large quantities of hydrogen. The main challenge is cost relative to other energy sources and fuels.
Mineral extraction
The ocean contains 57 trace elements in salts and other forms and dissolved in solution. In the past, most economic analyses concluded that mining the ocean for trace elements would be unprofitable, in part because of the energy required to pump the water. Mining generally targets minerals that occur in high concentrations, and can be extracted easily, such as magnesium. With OTEC plants supplying water, the only cost is for extraction. The Japanese investigated the possibility of extracting uranium and found developments in other technologies (especially materials sciences) were improving the prospects.
Political concerns
Because OTEC facilities are more-or-less stationary surface platforms, their exact location and legal status may be affected by the United Nations Convention on the Law of the Sea treaty (UNCLOS). This treaty grants coastal nations 3-, 12-, and 200-mile (320 km) zones of varying legal authority from land, creating potential conflicts and regulatory barriers. OTEC plants and similar structures would be considered artificial islands under the treaty, giving them no independent legal status. OTEC plants could be perceived as either a threat or potential partner to fisheries or to seabed mining operations controlled by the International Seabed Authority.
Cost and economics
For OTEC to be viable as a power source, the technology must have tax and subsidy treatment similar to competing energy sources. Because OTEC systems have not yet been widely deployed, cost estimates are uncertain. One study estimates power generation costs as low as US $0.07 per kilowatt-hour, compared with $0.05 - $0.07 for subsidized wind systems.[23]
Beneficial factors that should be taken into account include OTEC's lack of waste products and fuel consumption, the area in which it is available, (often within 20° of the equator)[24] the geopolitical effects of petroleum dependence, compatibility with alternate forms of ocean power such as wave energy, tidal energy and methane hydrates, and supplemental uses for the seawater.[25]
Mathematics of OTEC
A rigorous treatment of OTEC reveals that a 20 °K temperature difference will provide as much energy as a hydroelectric plant with 34 m head for the same volume of water flow. The low temperature difference means that water volumes must be very large to extract useful amounts of heat and enormous heat exchangers must be employed compared to those used at a plant running with a larger temperature difference such as in conventional thermal power generation.[26]
Variation of ocean temperature with depth
The total insolation received by the oceans (covering 70% of the earth's surface, with clearness index of 0.5 and average energy retention of 15%) is 5.457 x e10 Megajoules/year (MJ/yr) x .7 x .5 x .15 = 2.87 x e10 MJ/yr
We can use Lambert's law to quantify the solar energy absorption by water,
where, y is the depth of water, I is intensity and μ is the absorption coefficient. Solving the above differential equation,
The absorption coefficient μ may range from 0.05 m−1 for very clear fresh water to 0.5 m−1 for very salty water.
Since the intensity falls exponentially with depth y, heat absorption is concentrated at the top layers. Typically in the tropics, surface temperature values are in excess of 25 °C (77 °F), while at 1 kilometer (0.62 mi), the temperature is about 5–10 °C (41–50 °F). The warmer (and hence lighter) waters at the surface means there are no thermal convection currents. Due to the small temperature gradients, heat transfer by conduction is too low to equalize the temperatures. The ocean is thus both a practically infinite heat source and a practically infinite heat sink.
This temperature difference varies with latitude and season, with the maximum in tropical, subtropical and equatorial waters. Hence the tropics are generally the best OTEC locations.
Open/Claude cycle
In this scheme, warm surface water at around 27 °C (81 °F) enters an evaporator at pressure slightly below the saturation pressures causing it to vaporize.
Where Hf is enthalpy of liquid water at the inlet temperature, T1.
This temporarily superheated water undergoes volume boiling as opposed to pool boiling in conventional boilers where the heating surface is in contact. Thus the water partially flashes to steam with two-phase equilibrium prevailing. Suppose that the pressure inside the evaporator is maintained at the saturation pressure, T2.
Here, x2 is the fraction of water by mass that vaporizes. The warm water mass flow rate per unit turbine mass flow rate is 1/x2.
The low pressure in the evaporator is maintained by a vacuum pump that also removes the dissolved non-condensable gases from the evaporator. The evaporator now contains a mixture of water and steam of very low vapor quality (steam content). The steam is separated from the water as saturated vapor. The remaining water is saturated and is discharged to the ocean in the open cycle. The steam is a low pressure/high specific volume working fluid. It expands in a special low pressure turbine.
Here, Hg corresponds to T2. For an ideal isentropic (reversible adiabatic) turbine,
The above equation corresponds to the temperature at the exhaust of the turbine, T5. x5,s is the mass fraction of vapor at state 5.
The enthalpy at T5 is,
This enthalpy is lower. The adiabatic reversible turbine work = H3-H5,s .
Actual turbine work WT = (H3-H5,s) x polytropic efficiency
The condenser temperature and pressure are lower. Since the turbine exhaust is to be discharged back into the ocean, a direct contact condenser is used to mix the exhaust with cold water, which results in a near-saturated water. That water is now discharged back to the ocean.
H6=Hf, at T5. T7 is the temperature of the exhaust mixed with cold sea water, as the vapour content now is negligible,
The temperature differences between stages include that between warm surface water and working steam, that between exhaust steam and cooling water, and that between cooling water reaching the condenser and deep water. These represent external irreversibilities that reduce the overall temperature difference.
The cold water flow rate per unit turbine mass flow rate,
Turbine mass flow rate, 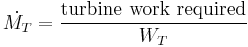
Warm water mass flow rate, 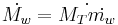
Cold water mass flow rate 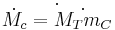
Closed/Anderson cycle
Developed starting in the 1960s by J. Hilbert Anderson of Sea Solar Power, Inc. In this cycle, QH is the heat transferred in the evaporator from the warm sea water to the working fluid. The working fluid exits the evaporator as a gas near its dew point.
The high-pressure, high-temperature gas then is expanded in the turbine to yield turbine work, WT. The working fluid is slightly superheated at the turbine exit and the turbine typically has an efficiency of 90% based on reversible, adiabatic expansion.
From the turbine exit, the working fluid enters the condenser where it rejects heat, -QC, to the cold sea water. The condensate is then compressed to the highest pressure in the cycle, requiring condensate pump work, WC. Thus, the Anderson closed cycle is a Rankine-type cycle similar to the conventional power plant steam cycle except that in the Anderson cycle the working fluid is never superheated more than a few degrees Fahrenheit. Owing to viscous effects, working fluid pressure drops in both the evaporator and the condenser. This pressure drop, which depends on the types of heat exchangers used, must be considered in final design calculations but is ignored here to simplify the analysis. Thus, the parasitic condensate pump work, WC, computed here will be lower than if the heat exchanger pressure drop was included. The major additional parasitic energy requirements in the OTEC plant are the cold water pump work, WCT, and the warm water pump work, WHT. Denoting all other parasitic energy requirements by WA, the net work from the OTEC plant, WNP is
The thermodynamic cycle undergone by the working fluid can be analyzed without detailed consideration of the parasitic energy requirements. From the first law of thermodynamics, the energy balance for the working fluid as the system is
where WN = WT + WC is the net work for the thermodynamic cycle. For the idealized case in which there is no working fluid pressure drop in the heat exchangers,
and
so that the net thermodynamic cycle work becomes
Subcooled liquid enters the evaporator. Due to the heat exchange with warm sea water, evaporation takes place and usually superheated vapor leaves the evaporator. This vapor drives the turbine and the 2-phase mixture enters the condenser. Usually, the subcooled liquid leaves the condenser and finally, this liquid is pumped to the evaporator completing a cycle.
Technical difficulties
Dissolved gases
The performance of direct contact heat exchangers operating at typical OTEC boundary conditions is important to the Claude cycle. Many early Claude cycle designs used a surface condenser since their performance was well understood. However, direct contact condensers offer significant disadvantages. As cold water rises in the intake pipe, the pressure decreases to the point where gas begins to evolve. If a significant amount of gas comes out of solution, placing a gas trap before the direct contact heat exchangers may be justified. Experiments simulating conditions in the warm water intake pipe indicated about 30% of the dissolved gas evolves in the top 8.5 meters (28 ft) of the tube. The trade-off between pre-dearation[27] of the seawater and expulsion of non-condensable gases from the condenser is dependent on the gas evolution dynamics, deaerator efficiency, head loss, vent compressor efficiency and parasitic power. Experimental results indicate vertical spout condensers perform some 30% better than falling jet types.
Microbial fouling
Because raw seawater must pass through the heat exchanger, care must be taken to maintain good thermal conductivity. Biofouling layers as thin as 25 to 50 micrometres (0.00098 to 0.0020 in) can degrade heat exchanger performance by as much as 50%.[2] A 1977 study in which mock heat exchangers were exposed to seawater for ten weeks concluded that although the level of microbial fouling was low, the thermal conductivity of the system was significantly impaired.[28] The apparent discrepancy between the level of fouling and the heat transfer impairment is the result of a thin layer of water trapped by the microbial growth on the surface of the heat exchanger.[28]
Another study concluded that fouling degrades performance over time, and determined that although regular brushing was able to remove most of the microbial layer, over time a tougher layer formed that could not be removed through simple brushing.[2] The study passed sponge rubber balls through the system. It concluded that although the ball treatment decreased the fouling rate it was not enough to completely halt growth and brushing was occasionally necessary to restore capacity. The microbes regrew more quickly later in the experiment (i.e. brushing became necessary more often) replicating the results of a previous study.[29] The increased growth rate after subsequent cleanings appears to result from selection pressure on the microbial colony.[29]
Continuous use of 1 hour per day and intermittent periods of free fouling and then chlorination periods (again 1 hour per day) were studied. Chlorination slowed but did not stop microbial growth; however chlorination levels of .1 mg per liter for 1 hour per day may prove effective for long term operation of a plant.[2] The study concluded that although microbial fouling was an issue for the warm surface water heat exchanger, the cold water heat exchanger suffered little or no biofouling and only minimal inorganic fouling.[2]
Besides water temperature, microbial fouling also depends on nutrient levels, with growth occurring faster in nutrient rich water.[30] The fouling rate also depends on the material used to construct the heat exchanger. Aluminium tubing slows the growth of microbial life, although the oxide layer which forms on the inside of the pipes complicates cleaning and leads to larger efficiency losses.[29] In contrast, titanium tubing allows biofouling to occur faster but cleaning is more effective than with aluminium.[29]
Sealing
The evaporator, turbine, and condenser operate in partial vacuum ranging from 3% to 1% of atmospheric pressure. The system must be carefully sealed to prevent in-leakage of atmospheric air that can degrade or shut down operation. In closed-cycle OTEC, the specific volume of low-pressure steam is very large compared to that of the pressurized working fluid. Components must have large flow areas to ensure steam velocities do not attain excessively high values.
Parasitic power consumption by exhaust compressor
An approach for reducing the exhaust compressor parasitic power loss is as follows. After most of the steam has been condensed by spout condensers, the non-condensible gas steam mixture is passed through a counter current region which increases the gas-steam reaction by a factor of five. The result is an 80% reduction in the exhaust pumping power requirements.
Cold air/warm water conversion
In winter in coastal Arctic locations, seawater can be 40 °C (72 °F) warmer than ambient air temperature. Closed-cycle systems could exploit the air-water temperature difference. Eliminating seawater extraction pipes might make a system based on this concept less expensive than OTEC. This technology is due to H. Barjot, who suggested butane as cryogen, because of its freezing point of −0.5 °C (31.1 °F) and its non-solubility in water.[31] Assuming a level of efficiency of realistic 4 %, calculations show that the amount of energy generated with one cubic meter water at a temperature of 2 °C (36 °F) in a place with an air temperature of −22 °C (−8 °F) equals the amount of energy generated by letting this cubic meter water run through a hydroelectric plant of 4000 feet (1,200 m) height.[32]
Barjot Polar Power Plants could be located on island in the polar region or designed as swimming barges or platforms attached to the ice cap. The weather station Myggbuka at Greenlands east coast for example, which is only 2,100 km away from Glasgow, detects monthly mean temperatures below −15 °C (5 °F) during 6 winter months in the year.[33]
See also
Footnotes
- ^ a b c DiChristina, Mariette (May 1995). "Sea Power". Popular Science: 70–73. http://books.google.co.uk/books?id=4jETasQk7aoC&pg=PA73&dq=otec&hl=en&ei=qFXATtD1GIamhAfNs53IBA&sa=X&oi=book_result&ct=result&resnum=1&ved=0CDgQ6AEwAA#v=onepage&q=otec&f=false. Retrieved Nov 2011.
- ^ a b c d e Berger LR, Berger JA (June 1986). "Countermeasures to Microbiofouling in Simulated Ocean Thermal Energy Conversion Heat Exchangers with Surface and Deep Ocean Waters in Hawaii". Appl. Environ. Microbiol. 51 (6): 1186–1198. PMC 239043. PMID 16347076. http://aem.asm.org/cgi/pmidlookup?view=long&pmid=16347076.
- ^ Chiles, James (Winter 2009). "The Other Renewable Energy". Invention and Technology 23 (4): 24–35.
- ^ "Power from the Sea" Popular Mechanics, December 1930, pp 881-882 detail article and photos of Cuban power plant
- ^ a b c d Takahashi, Masayuki Mac; Translated by: Kitazawa, Kazuhiro and Snowden, Paul (2000) [1991]. Deep Ocean Water as Our Next Natural Resource. Tokyo, Japan: Terra Scientific Publishing Company. ISBN 4-88704-125-x. http://www.terrapub.co.jp/e-library/dow/index.html.
- ^ Tesla, Nikola (December 1931). "On Future Motive Power". Everyday Science and Mechanics: 230–236. http://www.tesla.hu/tesla/articles/19311200/index.htm.
- ^ US patent 3312054, J.H. Anderson, "Sea Water Power Plant", issued 1967-04-04
- ^ a b Bruch, Vicki L. (April 1994) (PDF). An Assessment of Research and Development Leadership in Ocean Energy Technologies. SAND93-3946. Sandia National Laboratories: Energy Policy and Planning Department. http://www.osti.gov/bridge/servlets/purl/10154003-z9rVWD/native/10154003.PDF.
- ^ Mitsui, T.; Ito, F.; Seya, Y.; Nakamoto, Y. (September 1983). "Outline of the 100 kW OTEC Pilot Plant in the Republic of Nauru". IEEE Transactions on Power Apparatus and Systems PAS-102 (9): 3167–3171. doi:10.1109/TPAS.1983.318124. http://library.greenocean.org/oteclibrary/otecdesigns/OTEC-nauru.pdf/view.
- ^ "Average Retail Price of Electricity to Ultimate Customers by End-Use Sector, by State". Energy Information Administration. September 2007. http://www.eia.doe.gov/cneaf/electricity/epm/table5_6_a.html.
- ^ "Deep Pipelines for Ocean Thermal Energy Conversion". http://www.makai.com/p-otec.htm. Retrieved 2009-02-16.
- ^ US patent 4311012, Warren T. Finley, "Method and apparatus for transferring cold seawater upward from the lower depths of the ocean to improve the efficiency of ocean thermal energy conversion systems", issued 1982-01-19
- ^ Trimble, L.C.; Owens, W.L. (1980). "Review of mini-OTEC performance". Energy to the 21st century; Proceedings of the Fifteenth Intersociety Energy Conversion Engineering Conference 2: 1331–1338. Bibcode 1980iece....2.1331T.
- ^ Vega, L.A. (1999). "Open Cycle OTEC". OTEC News. The GreenOcean Project. http://www.otecnews.org/articles/vega/05_open_cycle.html. Retrieved 4 February 2011.
- ^ a b "Achievements in OTEC Technology". National Renewable Energy Laboratory. http://www.nrel.gov/otec/achievements.html.
- ^ "Lockheed Martin awarded another $4.4M for OTEC work in Hawaii". November 22, 2010. http://www.bizjournals.com/pacific/news/2010/11/22/lockheed-martin-awarded-another-44m.html. Retrieved December, 2010.
- ^ Coxworth, Ben (November 26, 2010). "More funds for Hawaii's Ocean Thermal Energy Conversion plant". http://www.gizmag.com/lockheed-martin-otec-hawaii/17081/. Retrieved December, 2010.
- ^ U.S. Department of Energy, 1989)
- ^ "YouTube video on the OTEC air-conditioning system used at the InterContinental Resort and Thalasso-Spa on the island of Bora Bora". http://www.youtube.com/watch?v=zTGvPrrkVAA. Retrieved 2007-05-28.
- ^ us 7069689
- ^ Block and Lalenzuela 1985
- ^ US 7726138
- ^ http://www.pichtr.org/Luis_Vega_OTEC_Summary.pdf
- ^ http://www.nrel.gov/otec/markets.html
- ^ http://www.nrel.gov/otec/
- ^ Da Rosa, Aldo Vieira (2009). "4". Fundamentals of renewable energy processes. Academic Press. pp. 139 to 152. ISBN 0123746396. http://books.google.co.uk/books?id=vDuec62ube8C&pg=PA146&dq=otec&hl=en&ei=SFjATqf3OsrDhAe76qDABA&sa=X&oi=book_result&ct=result&resnum=4&ved=0CEEQ6AEwAw#v=onepage&q=otec&f=false.
- ^ deaeration
- ^ a b Aftring RP, Taylor BF (October 1979). "Assessment of Microbial Fouling in an Ocean Thermal Energy Conversion Experiment". Appl. Environ. Microbiol. 38 (4): 734–739. PMC 243568. PMID 16345450. http://aem.asm.org/cgi/pmidlookup?view=long&pmid=16345450.
- ^ a b c d Nickels JS, Bobbie RJ, Lott DF, Martz RF, Benson PH, White DC (June 1981). "Effect of Manual Brush Cleaning on Biomass and Community Structure of Microfouling Film Formed on Aluminum and Titanium Surfaces Exposed to Rapidly Flowing Seawater". Appl. Environ. Microbiol. 41 (6): 1442–1453. PMC 243937. PMID 16345798. http://aem.asm.org/cgi/pmidlookup?view=long&pmid=16345798.
- ^ Trulear, MG; Characklis, WG (September 1982). "Dynamics of Biofilm Processes". Journal of the Water Pollution Control Federation 54 (9): 1288–1301. http://md1.csa.com/partners/viewrecord.php?collection=ENV&recid=8301080&uid=792056511&q=&uid=792056511&q=&uid=792056511&setcookie=yes.
- ^ "Science: Cold Power". Time. 1929-04-22. http://www.time.com/time/magazine/article/0,9171,751867,00.html.
- ^ http://www.buch-der-synergie.de/c_neu_html/c_06_10_wasser_temperaturgradient.htm
- ^ http://www.globalbioclimatics.org/station/de-myggb.htm
References
- http://www.lockheedmartin.com/products/OTEC/index.html
- http://www.makai.com/e-otec.htm
- http://www.ocees.com
Sources
- Renewable Energy From The Ocean - A Guide To OTEC, William H. Avery, Chih Wu, Oxford University Press, 1994. Covers the OTEC work done at the Johns Hopkins Applied Physics Laboratory from 1970–1985 in conjunction with the Department of Energy and other firms.
External links
- Sejarah Perkembangan Teknologi OTEC [1]
- - Ocean Energy Council: How does OTEC work?
- nrel.gov - what is OTEC?
- US Department of Energy, Information Resources
- Wired Magazine's interview with John Piña Craven on the future of OTEC
- OTEC News - a news site about OTEC
- 2007 edition of the Survey of Energy Resources produced by the World Energy Council
- The Green Ocean Project - OTEC Library
- The Green Ocean Project (CO2 release)
- Plumbing the oceans could bring limitless clean energy
- [2] Maximum water flow capacity of steel pipes - dimensions ranging 2 - 24 inches
The Engineering Toolbox. for other types of ocean energy go to ((tidal energy))
|
||||||||||||||||||||